Abstract
Background: Survival rates for pediatric Hodgkin lymphoma (HL) exceed 95% with contemporary therapy. To reduce long-term morbidity while maintaining excellent survival, risk-adapted therapy including tailored use of radiation has been developed. Studies of 5-year survivors and cohorts treated in the 1970s-1990s have shown pediatric HL survivors are at increased risk for treatment-related chronic health conditions, including subsequent malignancies, cardiopulmonary disease, thyroid dysfunction, and infertility. However, little is known about chronic conditions associated with contemporary therapy presenting during the first 5 years from diagnosis (early outcomes). The purpose of this study was to analyze survival and early outcomes of pediatric HL patients treated with contemporary therapy.
Methods: We conducted a retrospective review of HL patients diagnosed <21 years of age and treated at our institution from 2011-2014. Three patients treated with surgery only were excluded. Three-year overall (OS) and event-free (EFS) survival were calculated with Kaplan Meier statistics using SAS 9.4. Results of screening (per Children's Oncology Group and Institutional Fertility Preservation Screening Guidelines) for targeted toxicities that developed between 2 and 5 years from diagnosis were identified and graded per CTCAE criteria. Censoring occurred at date of death, 5 years after diagnosis, or December 31, 2016. Data from the last collection point were used for prevalence calculations in cases with multiple evaluations. Descriptive statistics were calculated for the cohort.
Results: We identified 62 patients (52% male; 39% non-Hispanic white; mean age at diagnosis 13.6 ± 3.6 years) with a median time since diagnosis of 3.3 years (range 0.06-5.0). Initial treatment included: 49 (79%) chemotherapy only and 13 (21%) multimodality treatment. Chemotherapy comprised ABVE-PC (56%), BEACOPP (19%), COPP/ABV (16%), or other (8%). Four patients with relapsed/refractory disease received salvage treatment including chemotherapy (n=1), multimodality therapy (n=1), or multimodality treatment including stem cell transplant (SCT; n=2). One patient remained on treatment at the time of censoring.
The 3-year OS across all stages was 98.4% with an EFS of 91.9% (89.7% chemotherapy only, 100% multimodality treatment). The one mortality was due to toxic death during chemotherapy.
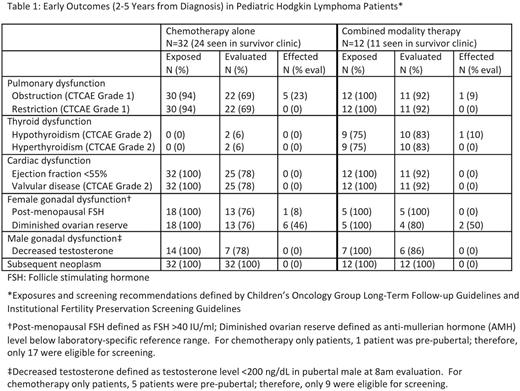
At our institution, patients are referred to survivor clinic at 2 years from completion of therapy. Of the 44 patients eligible for survivor clinic, 35 (80%) attended during the study period, 5 (11%) were seen after the study period, 3 (7%) were lost to follow-up, and 1 (2%) was followed by the primary oncologist only. Early outcomes for patients who received exposure-based screening are listed in Table 1. No patients developed pulmonary dysfunction greater than CTCAE Grade 1, hyperthyroidism, thyroid nodules, cardiac dysfunction, or a subsequent neoplasm during the study period.
Anti-mullerian hormone (AMH) below the normal range was found in all 8 evaluated females who received procarbazine-based regimens (BEACOPP or COPP/ABV; 6 received chemotherapy alone, 2 received chemotherapy with supradiaphragmatic radiation). All 8 females who received ABVE-PC with or without radiation (1 abdominal) had normal AMH levels. No males had abnormal gonadotropins.
Conclusions: With the incorporation of novel agents in HL therapy, defining the baseline early outcomes of contemporary regimens is germane to future comparative medicine initiatives. This study is the first to evaluate early outcomes in pediatric HL survivors. While the cohort is young, the results indicate contemporary chemotherapy and a lower rate of radiation therapy utilization leads to excellent 3 year survival rates with minimal early toxicities. Females treated with procarbazine-based regimens are at increased risk for diminished ovarian reserve compared to those receiving ABVE-PC and should be prioritized for fertility preservation approaches prior to initiation of therapy. We plan to further expand our experience to include patients diagnosed since 2005 and evaluate the relationship between cumulative alkylating agent exposure and female gonadal dysfunction.
[Chemotherapy abbreviations: (A) Adriamycin, (B) Bleomycin, (C) Cyclophosphamide, (E) Etoposide, (O) Vincristine, (P) Prednisone, (P) Procarbazine, (V) Vinblastine]
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.